(OBP-301)
(OBP-301)
- Outline / Target indication / Current status
- Preclinical studies and proof of concept
- Clinical Development Strategy and Program
The Target: Telomerase – essential to cancer cell immortalization
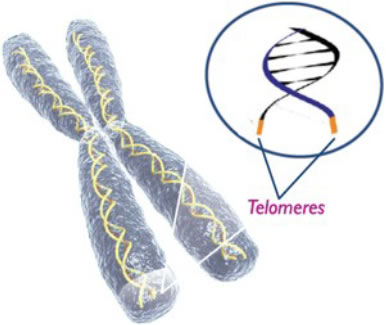
Telomerase is a ribonucleoprotein complex that is responsible for adding TTAGGG repeats onto the 3’ends of chromosomes and thus is crucial for the survival of cancer cells
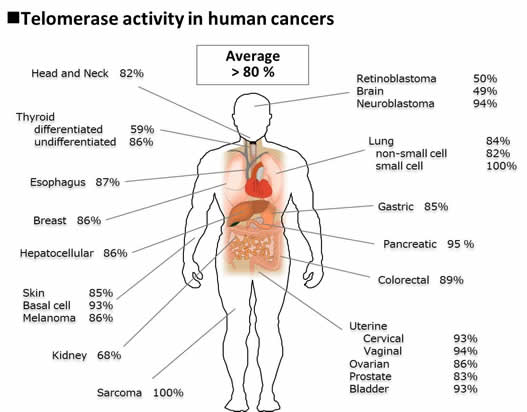
The majority (>80%) of malignant tumors express telomerase activity, whereas telomerase is strongly repressed in most normal somatic tissues>br>Telomerase may be a plausible target for cancer diagnosis and therapy
Telomelysin (OBP-301) is a novel oncolytic adenovirus
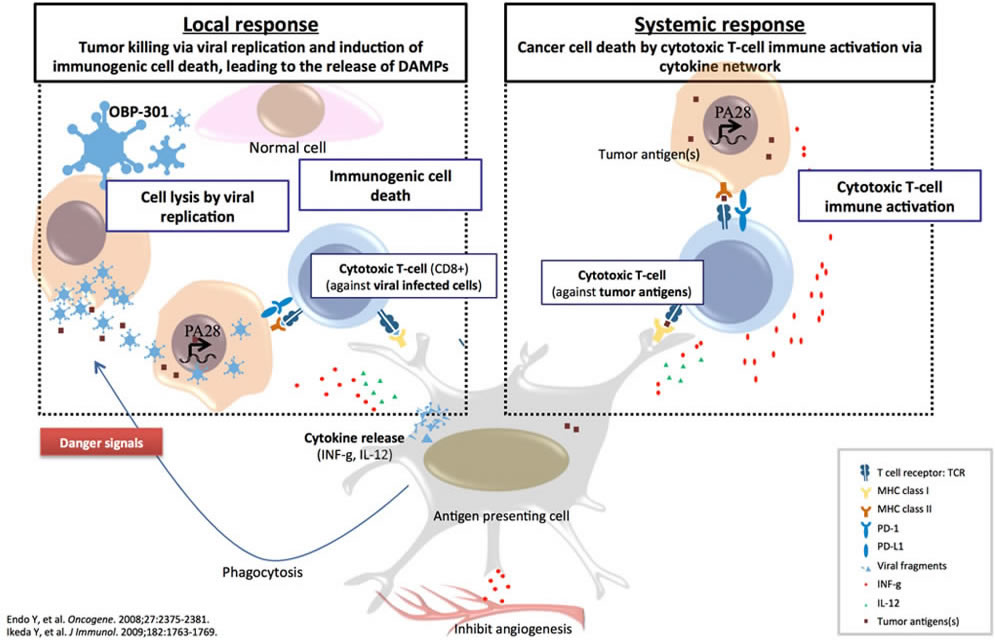
Oncolytic virus selectively kills tumors, releasing the full array of neoantigens in an inflamed tumor environment leading to tumor cell death
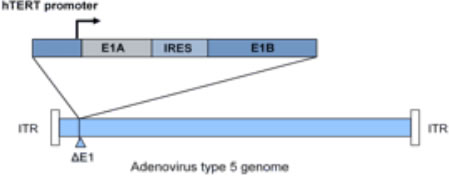
Telomelysin is an intratumoral injectable virotherapy with the ability to replicate within cancer cells – causing cancer cell lysis
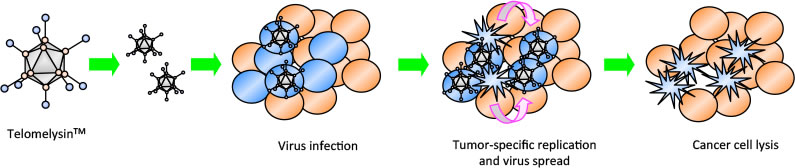
Telomelysin (OBP-301) Proposed Mechanism of Action
OBP-301 selectively kills tumors, releasing the full array of neoantigens in an inflamed tumor environment leading to tumor cell death
Phase I Study in advanced solid tumors Conclusions: Telomelysin monotherapy is well-tolerated with promising clinical benefit
- Single and multiple doses were well tolerated (up to 5 x 1012 VP)
- Most adverse events were mild to moderate in severity and transient Injection site reactions, fever, chills
- No liver toxicity
- Transient lymphopenia was observed 24 hours after injection
- Measurable levels in plasma after 1-3 hours
- Patients had measurable levels at 7 and 14 days after treatment
- Viral DNA was detected in plasma of some patients at 7 and 14 days after injection.
- Indication of reduction in area of treated tumor in some patients
- No patient showed any local progressive disease
- Abscopal effect was observed in melanoma patients
- Immune cell infiltration was observed in injected site
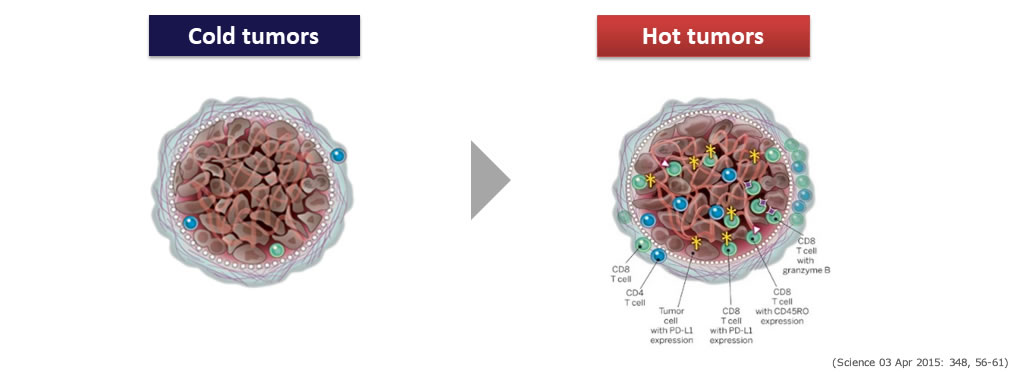
As an oncolytic virus, Telomelysin can be combined with other modalities to transform cold tumors to hot tumors
Radiotherapy
Telomelysin enhances radiosensitization by blocking DNA repair
In mouse models of human cancer, Telomelysin induced a synergistic antitumor effect due to tumor cell-specific radiosensitization (combined with regional irradiation)Immuno checkpoint inhibition
Telomelysin may be synergistic with anti-PD-1 antibodies by enhancing tumor immunogenicity
In preclinical murine models, Telomelysin induced immunogenic cell death, sensitizing non-immunogenic gastrointestinal tumors to PD-1 Ab
Summary of combination study with RT: Telomelysin is well-tolerated with impressive CR rate in patients with unresectable esophageal cancer
- Multiple doses of Telomelysin were well tolerated (1 x 1010~1012 VP)
- Most adverse events were mild to moderate in severity and transient
- Transient lymphocytopenia was seen in all patients
- Plasma, sputum, saliva, and urine specimens were collected for viral DNA
- Virus shedding in the body fluids could not be determined
- Telomelysin DNA could be transiently detected in plasma
- Indication of reduction in area of treated tumor in some patients 8 of 13 CRs
- Pathological responses were also observed in some patients