Outline
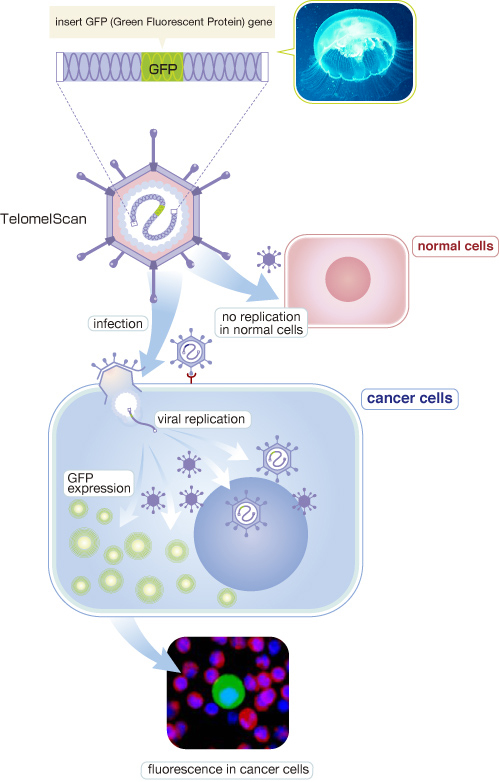
OBP-401 or TelomeScan is a genetically engineered adenovirus type 5 to detect tumor cells. TelomeScan has a human Telomerase Reverse Transcriptase (hTERT) gene promoter sequence in the upstream region of virus E1 gene which is responsible for replication of adenovirus, and a Green Fluorescent Protein (GFP) gene in E3 region under the control by cytomegalovirus promoter. TERT is a critical protein component of telomerase enzyme complex, and its expression is normally restricted in germ line cells or few stem cells including hematopoietic stem cells. Cancer cells are essentially acquired immortalized nature and express strong telomerase activity. Thus, OBP-401 can replicate only in telomerase activity-positive cells such as cancer cells by hTERT promoter activity-dependent E1/E2 gene expression, but not in telomerase activity-negative normal cells. Along with the increase in virus replication, concurrently-expressed GFP accumulates in hTERT-positive cells.
By utilizing OBP-401 ex vivo, it is possible to detect living circulating tumor cells (CTCs) in blood from cancer patients with high sensitivity and specificity. CTCs detection would be able to offer a tool for early detection of cancer, monitoring of therapeutic efficacy, monitoring of tumor recurrence, and selection of appropriate therapy to patients.
OBP-1101 is second generation of TelomeScan. Since TelomeScan has two issues to be improved, i.e. false negative and false positive. TelomeScan cannot be infected to some kind of cancer cells which lack adenovirus receptor "CAR" (coxackie-adenovirus receptor) on the cell surface. CAR-lacking cancer cells are regarded as "false negative". In contrast, TelomeScan generates false positive in some white blood cells having hTERT activity. To dissolve these issues, two genetic modifications including change in fibre gene and addition of four tandem-arrayed miR142-3p target sequences were added on OBP-401 vector sequence.
Target indication
Detection of circulating tumor cells (CTCs)
Current status
- For progress of development, refer to "Outline of Our Pipeline: Progress chart of pipeline.
- For details, refer to "Research and Development Activities" in the latest financial statements.