OBP-601 (Censavudine)
新たな疾患領域への応用に期待
概要
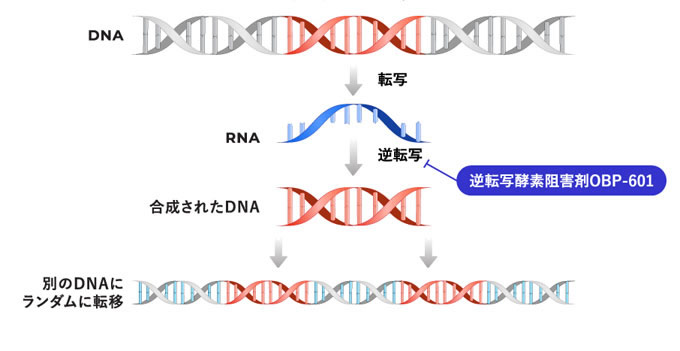
OBP-601(Censavudine)は、RNAからDNAへの逆転写を阻害する核酸系逆転写酵素阻害剤(NRTI)です。
OBP-601は、重症感染症のHIV治療薬として開発することを目的に2006年にYale大学から導入し、2010年から2014年にかけてBristol-Myers Squibb Co.がPhase2臨床試験の完了まで開発を進めてきました。その後、HIV治療薬のマーケットが飽和状態となり、新規ライセンスの可能性が低い状況の中、感染症領域以外でのOBP-601の新規ライセンス契約締結に向けたビジネス活動を積極的に推進しました。
その結果、逆転写酵素阻害作用を重篤な疾患へ応用することを目的に設立されたTransposon Therapeutics, Inc.(以下「Transposon社」)との間で総額3億ドル超の新規ライセンス契約を2020年6月に締結しました。今後の開発はTransposon社が全世界で実施することになっています。
開発の状況
進捗に関しては、「パイプラインの概要:パイプラインの進捗一覧 」をご参照ください。
開発進捗に関する詳細は、決算短信末尾にあります「研究開発状況」をご参照ください。
特許の概況
センサブジンは、平成29年1月末時点で日本を含む世界13ヶ国での特許取得が完了しています。
- (特許取得済みの国)
- 日本・米国・オーストラリア・カナダ・中国・香港・ロシア・イスラエル・韓国・メキシコ・ニュージーランド・ポーランド・シンガポール
詳しくはこちらもご参照ください。